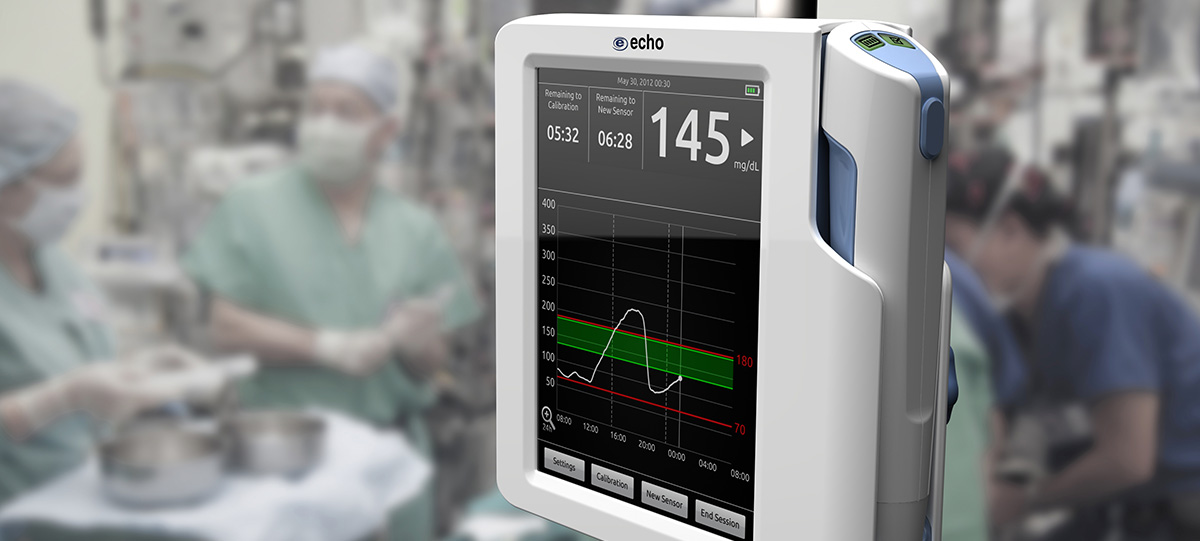
Ruggedized Design
NanoDx Analyzer
Getting Results, Faster

We support research and testing activities with a spacious on-site observation laboratory equipped with audio and video recording capabilities. The laboratory can be readily configured to resemble a conference room, a living room, or a hospital room. Our clients enjoy viewing first-hand users’ impressions from behind a one-way mirror.